Ephrin A5
| EFNA5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | EFNA5, AF1, EFL5, EPLG7, GLC1M, LERK7, RAGS, ephrin A5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 601535; MGI: 107444; HomoloGene: 1482; GeneCards: EFNA5; OMA:EFNA5 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Ephrin A5 is a protein that in humans is encoded by the EFNA5 gene.[5][6][7]
Ephrin A5 is a glycosylphosphatidylinositol (GPI)-anchored protein of the ephrin-A subclass of ephrin ligands that binds to the EphA subclass of Eph receptors. Ephrin A5 has also been shown to bind to the EphB2 receptor.[8]
Reverse signaling in growth cone survival
"Reverse" signaling is one unique property of ephrin ligands that allows for the transmission of an intracellular signal in ephrin-expressing cells that is distinct from the signal transmitted in Eph receptor-expressing cells. Although the mechanism of "reverse" signaling by ephrin-As is not well understood, it is relatively surprising considering that ephrin-A ligands are attached to the cell membrane solely by a GPI linkage and unlike ephrin-Bs, lack a potential intracellular signaling domain. Nonetheless, certain ephrin-A ligands are known to initiate reverse signaling cascades like ephrin A5, which has been shown to stimulate the spreading of growth cones in cultures of mouse spinal motor neurons.[9] Reverse signaling by ephrin A5 was demonstrated to be GPI-dependent as the elimination of all GPI linkages by the application of a phosphatidlyinositol-specific phospholipase C abolished the positive effects of ephrin A5 on growth cone spreading. Additionally, EphA receptors were shown to exert opposite effects on motor neuron growth cones by reducing growth cone size.
Formation of the retinotopic map
This finding that ephrin A5 promotes growth cone survival that is opposite of EphA signaling and mediated directly by ephrin A5 reverse signaling has important implications for axon guidance as it provides a mechanism by which migrating axons expressing EphAs would preferentially avoid ephrin A5 expressing cells and possibly migrate towards cells with lower expression of ephrin A5.[9] This mechanism is in fact the same one that mediates the guidance of retinal ganglion cells to distinct regions in the superior colliculus during the formation of the retinotopic map. High ephrin A5 expression on cells in the posterior region of the SC bind to EphAs expressed in RGCs migrating from the temporal retina, inducing growth cone collapse and repelling these RGCs away from the posterior SC towards a region of low ephrin A5 expression in the anterior SC.[10]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000184349 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000048915 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Cerretti DP, Copeland NG, Gilbert DJ, Jenkins NA, Kuefer MU, Valentine V, Shapiro DN, Cui X, Morris SW (Sep 1996). "The gene encoding LERK-7 (EPLG7, Epl7), a ligand for the Eph-related receptor tyrosine kinases, maps to human chromosome 5 at band q21 and to mouse chromosome 17". Genomics. 35 (2): 376–9. doi:10.1006/geno.1996.0371. PMID 8661153.
- ^ Kozlosky CJ, VandenBos T, Park L, Cerretti DP, Carpenter MK (Oct 1997). "LERK-7: a ligand of the Eph-related kinases is developmentally regulated in the brain". Cytokine. 9 (8): 540–9. doi:10.1006/cyto.1997.0199. PMID 9245480.
- ^ "Entrez Gene: EFNA5 ephrin-A5".
- ^ Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB (May 2004). "Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling". Nat. Neurosci. 7 (5): 501–9. doi:10.1038/nn1237. PMID 15107857. S2CID 15643420.
- ^ a b Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL (April 2005). "Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains". Cell. 121 (1): 127–39. doi:10.1016/j.cell.2005.01.020. PMID 15820684. S2CID 16818608.
- ^ Drescher U, Kremoser C, Handwerker C, Löschinger J, Noda M, Bonhoeffer F (August 1995). "In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases". Cell. 82 (3): 359–70. doi:10.1016/0092-8674(95)90425-5. PMID 7634326. S2CID 2537692.
Further reading
- Flanagan JG, Vanderhaeghen P (1998). "The ephrins and Eph receptors in neural development". Annu. Rev. Neurosci. 21: 309–45. doi:10.1146/annurev.neuro.21.1.309. PMID 9530499. S2CID 1278600.
- Zhou R (1998). "The Eph family receptors and ligands". Pharmacol. Ther. 77 (3): 151–81. doi:10.1016/S0163-7258(97)00112-5. PMID 9576626.
- Holder N, Klein R (1999). "Eph receptors and ephrins: effectors of morphogenesis". Development. 126 (10): 2033–44. doi:10.1242/dev.126.10.2033. PMID 10207129.
- Wilkinson DG (2000). "Eph receptors and ephrins: regulators of guidance and assembly". Int. Rev. Cytol. International Review of Cytology. 196: 177–244. doi:10.1016/S0074-7696(00)96005-4. ISBN 978-0-12-364600-2. PMID 10730216.
- Xu Q, Mellitzer G, Wilkinson DG (2001). "Roles of Eph receptors and ephrins in segmental patterning". Philos. Trans. R. Soc. Lond. B Biol. Sci. 355 (1399): 993–1002. doi:10.1098/rstb.2000.0635. PMC 1692797. PMID 11128993.
- Wilkinson DG (2001). "Multiple roles of EPH receptors and ephrins in neural development". Nat. Rev. Neurosci. 2 (3): 155–64. doi:10.1038/35058515. PMID 11256076. S2CID 205014301.
- Winslow JW, Moran P, Valverde J, et al. (1995). "Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation". Neuron. 14 (5): 973–81. doi:10.1016/0896-6273(95)90335-6. PMID 7748564. S2CID 18373575.
- Gale NW, Holland SJ, Valenzuela DM, et al. (1996). "Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis". Neuron. 17 (1): 9–19. doi:10.1016/S0896-6273(00)80276-7. PMID 8755474. S2CID 1075856.
- Lackmann M, Mann RJ, Kravets L, et al. (1997). "Ligand for EPH-related kinase (LERK) 7 is the preferred high affinity ligand for the HEK receptor". J. Biol. Chem. 272 (26): 16521–30. doi:10.1074/jbc.272.26.16521. PMID 9195962.
- Ephnomenclaturecommittee (1997). "Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee". Cell. 90 (3): 403–4. doi:10.1016/S0092-8674(00)80500-0. PMID 9267020. S2CID 26773768.
- Ciossek T, Monschau B, Kremoser C, et al. (1998). "Eph receptor-ligand interactions are necessary for guidance of retinal ganglion cell axons in vitro". Eur. J. Neurosci. 10 (5): 1574–80. doi:10.1046/j.1460-9568.1998.00180.x. PMID 9751130. S2CID 20470923.
- Janis LS, Cassidy RM, Kromer LF (1999). "Ephrin-A binding and EphA receptor expression delineate the matrix compartment of the striatum". J. Neurosci. 19 (12): 4962–71. doi:10.1523/JNEUROSCI.19-12-04962.1999. PMC 6782661. PMID 10366629.
- Gerlai R, Shinsky N, Shih A, et al. (1999). "Regulation of learning by EphA receptors: a protein targeting study". J. Neurosci. 19 (21): 9538–49. doi:10.1523/JNEUROSCI.19-21-09538.1999. PMC 6782889. PMID 10531456.
- Davy A, Gale NW, Murray EW, et al. (2000). "Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion". Genes Dev. 13 (23): 3125–35. doi:10.1101/gad.13.23.3125. PMC 317175. PMID 10601038.
External links
- Overview of all the structural information available in the PDB for UniProt: P52803 (Human Ephrin-A5) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: O08543 (Mouse Ephrin-A5) at the PDBe-KB.
- v
- t
- e
-
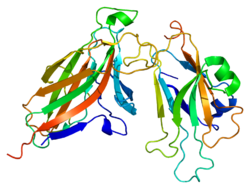
 1shw: EphB2 / EphrinA5 Complex Structure
1shw: EphB2 / EphrinA5 Complex Structure -
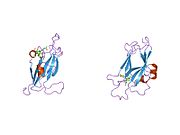 1shx: Ephrin A5 ligand structure
1shx: Ephrin A5 ligand structure